I have to read a lot of science papers. Science papers are not fun to read because they are confusing. They don’t
have to be confusing, but unfortunately most of them turn out that way. This is something I have a beef with. I want to know what these science papers are saying, I want to know what your models do or what your data shows, but I can’t do that when you don’t paint a clear picture for me. I could go on and on about my hang-ups with this topic, but instead I would like to describe to you a science paper in my own words. Today, we take a look at a paper by Boyd, et al. that was published in 2007 in Science, titled: “Mesoscale Iron Enrichment Experiments 1993 – 2005: Synthesis and Future Directions”. I think by doing this, I will digest this paper a little bit better and you’ll get to find out some interesting things pertaining to the field of oceanic iron research.
So what’s the deal with iron anyways? Well, phytoplanktons in the ocean need iron just like you and I. They become wimpy and anemic without iron just like we would. This is a big, BIG topic right now seeing as we (humans) would very much like to increase the amount of primary production in the oceans. Primary production, that is to say photosynthesis, is one way that we might save our asses from the looming Greenhouse Apocalypse because plants remove CO2 from the atmosphere and turn it into oxygen. Yay plants!
The ocean is FILLED with plants in the form of phytoplankton. The problem is that phytoplankton don’t grow everywhere, they can only grow in places that have the nutrients they need. Nutrients like nitrate, phosphate, silica. There are a couple places on Earth where it seems like conditions are perfect for phytoplankton growth, but nothing is going on. These places are called High Nutrient Low Productivity zones or HNLP zones. The Southern Ocean is a HNLP zone.
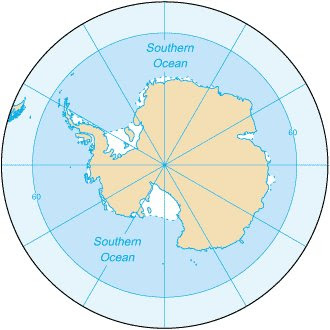
Conditions should be perfect for primary production in the Southern Ocean. Lots of cold water, lots of nutrients, plenty of mixing, and sunlight for part of the year. Problem is those phytoplanktons don’t grow there. Why?
A guy by the name of John Martin proposed that phytoplankton don’t grow in these regions because they are limited by iron. I should take this opportunity to say that iron is a really, really, REALLY hard thing to measure in the oceans because you so many possibilities for contamination. It took years for researchers to figure out why their iron numbers looked so wonky. The problem is that iron is everywhere – your boat, your collection devices, your bottles, your cables, your hands…you yourselves are a tremendous source of iron. When you’re measuring iron in parts per billion concentrations, every little bit counts. This problem was somewhat remedied by instituting clean rooms and anal-retentive practices that would limit the amount of iron contamination. But nothing is perfect.
Anywho, John Martin and those folks lucky enough to work with him set sail for the Southern Ocean armed with tons and tons of iron sulfate that they intended to dump in the water and see what might happen. What happened was exactly what he predicted – phytoplankton went apeshit. They grew like crazy and their bloom lasted for weeks. The picture below is a satellite image of chlorophyll (an indicator of phytoplankton) in the region where the ship dumped its load. You can see that the bloom pretty much follows the ship tracks.

(image from http://www.csa.com/discoveryguides/oceangard/images/soiree.jpg)
Is the story over? Fuck no, but I have to get back to work. Tune in on Friday for more on John Martin and oceanic iron fertilization…
PS: This article has a pretty cool description of John Martin and his research. Check him out. He was a cool dude who unfortunately passed away in 1993 right as his work was coming to fruition.









